
search
Your Partners in Unlocking New Possibilities. Meet Our Leadership at CPHI Frankfurt at Booth No. 5.0C84.
Book a MeetingMay 21, 2025
Antibody-drug conjugates (ADCs) represent a significant advancement in drug development, combining the targeting ability of antibodies with the cytotoxic potential of chemotherapy. These innovative therapies deliver potent drugs directly to cancer cells, minimizing damage to healthy tissues. The rising demand for ADC drug development is particularly evident in oncology, where they are proving effective against various tumors. This trend has increased interest in antibody-drug conjugate manufacturing and the specialized antibody-drug conjugate services supporting this process.
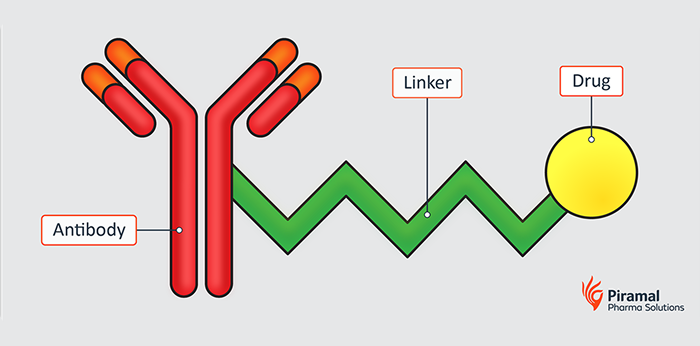

The mechanism of antibody-drug conjugation is central to its effectiveness in targeted drug delivery. ADC technology typically comprises three key components: the antibody, the linker, and the payload. The antibody binds specifically to antigens on cancer cells, allowing for precise targeting. Once internalized, the linker releases the cytotoxic payload, which leads to cell death.
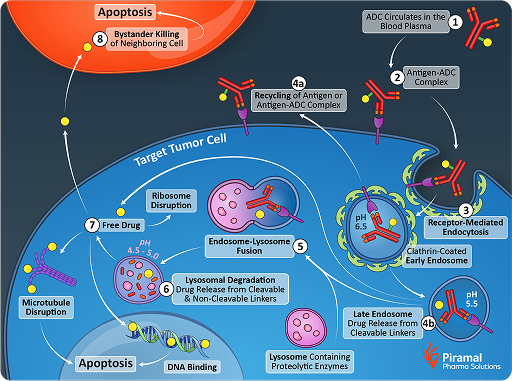
This precision targeting significantly reduces systemic toxicity compared to conventional chemotherapy, making ADCs a promising option for patients. Several approved ADC drugs have demonstrated substantial efficacy, showcasing their potential impact on treatment paradigms. This success underscores the importance of continued investment in antibody conjugates for various therapeutic areas beyond oncology.



The antibody-drug conjugate manufacturing process involves several critical steps, starting with antibody selection and culminating in the final formulation. Each step requires meticulous attention to detail to ensure the quality and effectiveness of the ADC.
ADC conjugation services utilize advanced bioconjugation chemistry to link antibodies to their payloads. This process must be optimized to maintain the integrity of the antibody while ensuring effective delivery of the cytotoxic drug. Purification, scalability, and stability testing are essential to guarantee that the final product is efficacious and aligned with regulatory standards.
Compliance with Good Manufacturing Practices (GMP) and regulatory guidelines from agencies like the FDA and EMA is crucial throughout manufacturing. This adherence ensures that ADCs are safe for patient use and effective in their therapeutic roles.
Despite their benefits, there are challenges associated with antibody-drug conjugate manufacturing. The complexity of ADC conjugation often leads to batch-to-batch consistency issues, which can impact product quality. Regulatory hurdles in ensuring the safety and efficacy of ADCs also pose significant challenges, requiring extensive testing and validation.
Scalability remains a concern as well. The production of ADCs often involves high production costs and complex supply chains, particularly for payload-linker systems. These factors can limit the availability of ADCs in the market, hindering patient access to these advanced therapies.


Antibody-drug conjugate development has a promising future, with advancements in site-specific ADC conjugation services enhancing precision and efficacy. Emerging next-generation payloads and linker technologies are under development to improve the therapeutic index of ADCs.
Moreover, the expansion of ADC technology beyond oncology into fields like immunotherapy and neurology is gaining traction. This diversification opens new avenues for treatment and presents opportunities for innovation. Growing partnerships between pharmaceutical companies and Contract Development and Manufacturing Organizations (CDMOs) are expected to facilitate large-scale production, ensuring these effective therapies become more widely available.
Antibody-drug conjugates represent a powerful tool in the fight against cancer and other diseases, combining targeted therapy with potent cytotoxic effects. To fully harness their potential, ongoing innovation in ADC manufacturing and conjugation technologies is essential. Continued investment in antibody-drug conjugate research and production capabilities will drive advancements in this promising field, ultimately benefiting patients and the healthcare system as a whole.
ADCelerate™
